In situ X-ray powder diffraction studies of hydrogen storage and release in the Li–N–H system†
Abstract
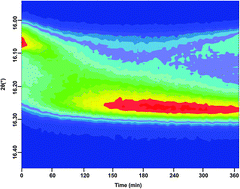
We report the experimental investigation of hydrogen storage and release in the lithium amide–lithium hydride composite (Li–N–H) system. Investigation of hydrogenation and dehydrogenation reactions of the system through in situ synchrotron X-ray powder diffraction experiments allowed for the observation of the formation and evolution of non-stoichiometric intermediate species of the form Li1+xNH2−x. This result is consistent with the proposed Frenkel-defect mechanism for these reactions. We observed capacity loss with decreasing temperature through decreased levels of lithium-rich (0.7 ≤ x ≤ 1.0) non-stoichiometric phases in the dehydrogenated material, but only minor changes due to multiple cycles at the same temperature. Annealing of dehydrogenated samples reveals the reduced stability of intermediate stoichiometry values (0.4 ≤ x ≤ 0.7) compared with the end member species: lithium amide (LiNH2) and lithium imide (Li2NH). Our results highlight the central role of ionic mobility in understanding temperature limitations, capacity loss and facile reversibility of the Li–N–H system.

 Please wait while we load your content...
Please wait while we load your content...