Protonic defects in yttria stabilized zirconia: incorporation, trapping and migration
Abstract
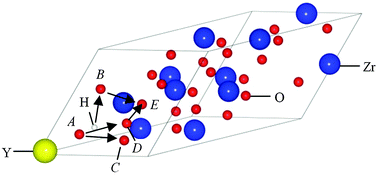
Both classical and quantum mechanical simulation techniques have been applied to investigate the incorporation, migration and potential binding of protonic defects in bulk yttria-stabilised zirconia (YSZ). The calculated redox reaction energies are found to be high, although the reduction energies are lower than those of bulk cubic ZrO2 and are shown to decrease further with increasing Y content. The hydration energies for YSZ are also lower than the values calculated for bulk ZrO2 and are found to be lowest when the oxygen ion is in close proximity to at least one Y ion. Strong binding (proton trapping) energies are observed between the protons and additional acceptor dopants including Sc, Yb and Gd. These energies are found to vary significantly depending on local configuration and again are generally lower than the values for ZrO2. Density functional theory (DFT) calculations are used to determine energy barriers for proton transfers via neighbouring oxygen ions (Grötthuss-type mechanism). Energy barriers of 0.32–0.42 eV are obtained for the pathways with the closest O–O interatomic distances and are found to be very comparable to well-established proton conducting materials.

 Please wait while we load your content...
Please wait while we load your content...