Complementarity of reaction force and electron localization function analyses of asynchronicity in bond formation in Diels–Alder reactions
Abstract
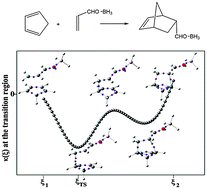
We have computationally compared three Diels–Alder cycloadditions involving cyclopentadiene and substituted ethylenes; one of the reactions is synchronous, while the others are slightly or highly asynchronous. Synchronicity and weak asynchronicity are characterized by the reaction force constant κ(ξ) having just a single minimum in the transition region along the intrinsic reaction coordinate ξ, while for high asynchronicity κ(ξ) has a negative maximum with minima on both sides. The electron localization function (ELF) shows that the features of κ(ξ) can be directly related to the formation of the new C–C bonds between the diene and the dienophile. There is thus a striking complementarity between κ(ξ) and ELF; κ(ξ) identifies the key points along ξ and ELF describes what is happening at those points.

 Please wait while we load your content...
Please wait while we load your content...