The pressure effect on thermotropic cubic phases of 1,2-bis(4′-n-alkoxybenzoyl)hydrazines†
Abstract
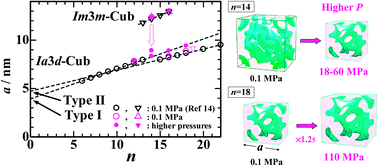
The effect of pressure on the nanostructure of a thermotropic cubic (Cub) mesogen 1,2-bis(4′-n-alkoxybenzoyl)hydrazine (BABH-n; n is the number of carbon atoms in the alkyl chain) was investigated under elevated pressures up to 140 MPa by an X-ray diffraction (XRD) technique. Four compounds, BABH-12, -14, -16 and -18, were examined and the type of Cub mesophase formed at ambient pressure is Ia3d for BABH-12 and -18, Im3m for BABH-14, and both for BABH-16. The high-pressure XRD enabled the discrimination of the Cub phase type in the low-pressure Cub phase regions of BABH-14 and BABH-16 and the revision of the phase diagrams reported previously. New insight in this work is changes in the lattice constant a of the Cub phases upon pressurization. The lattice constant a of the Im3m-Cub phase in BABH-14 decreases as only an exception, while those of the Ia3d-Cub phases in BABH-16 and -18 increased gradually, with increasing pressure, up to about 24 and 25% in the unit cell volume, respectively, in their optimal situations of pressure and temperature. The a values of the Ia3d-Cub phases in BABH-12, -14, -16 and -18 at elevated pressures were roughly on an extrapolated line of the a vs. n linear relationship determined for the corresponding data of the short-chain BABH-n (6 ≤ n ≤ 13) at ambient pressure. The pressure-induced expansion of the Ia3d-Cub lattice is well explained by reduced lateral expansion of a terminal alkyl chain and apparent reduction of the effective core size (from “double-layered core” to the “single-layered core” states).

 Please wait while we load your content...
Please wait while we load your content...