Reduction mechanisms of the CuO(111) surface through surface oxygen vacancy formation and hydrogen adsorption†
Abstract
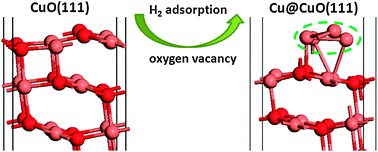
We studied the reduction of CuO(111) surface using density functional theory (DFT) with the generalized gradient approximation corrected for on-site Coulomb interactions (GGA + U) and screened hybrid DFT (HSE06 functional). The surface reduction process by oxygen vacancy formation and H2 adsorption on the CuO(111) surface is investigated as two different reduction mechanisms. It is found that both GGA + U and HSE06 predict the same trend in the relative stability of oxygen vacancies. We found that loss of the subsurface oxygen is initially thermodynamically favourable. As the oxygen vacancy concentration increases, mixture of subsurface and surface vacancies is energetically preferred over full reduction of the surface or subsurface monolayer. The reduction of Cu2+ to Cu+ is found to be more favourable than that of Cu+ to Cu0 in the most stable vacancy structures at all concentrations. Consistent with the oxygen vacancy calculations, H2 adsorption occurs initially on under-coordinated surface oxygen. Water molecules are formed upon the adsorption of H2 and this gives a mechanism for H2 reduction of CuO to Cu. Ab initio atomistic thermodynamics shows that reducing CuO to metallic Cu at the surface is more energetically difficult than in the bulk so that the surface oxide protects the bulk from reduction. Using H2 as the reducing agent, it is found that the CuO surface is reduced to Cu2O at approximately 360 K and that complete reduction from Cu2O to metallic Cu occurs at 780 K.

 Please wait while we load your content...
Please wait while we load your content...