Theoretical study of CO2 hydrogenation into formic acid on Lewis acid zeolites†
Abstract
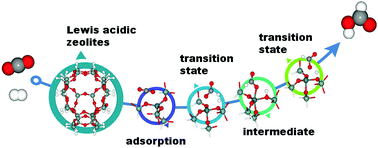
Conversion of carbon dioxide (CO2) to more valuable chemicals is nowadays receiving increasing attention from an environmental and industrial point of view. Herein, we computationally investigated CO2 hydrogenation to formic acid on Lewis acid zeolites by means of density functional theory (DFT) with the M06-L functional. The reaction proceeds in two steps, hydrogenation of CO2 to form the formate intermediate and hydrogen-abstraction to form formic acid. A defect zeolite seems to be favored over a perfect one, leading to its low rate determining step barrier of 5.2 kcal mol−1. We also considered the effect of the zeolite frameworks and found that the catalytic activities are in the order Sn-ZSM-5 > Sn-BEA > Sn-FAU. Finally, we performed catalytic activity screenings of tetravalent metals (Ge, Zr and Hf) substituted into the defect Sn-ZSM-5 zeolite. The order Hf > Zr > Sn > Ge was found based on the rate determining step activation energy. The difference in activation energy can be explained by the difference in charge transfer from the catalytic site to the reacting molecules.


 Please wait while we load your content...
Please wait while we load your content...