Molecular mechanism of activation of Burkholderia cepacia lipase at aqueous–organic interfaces
Abstract
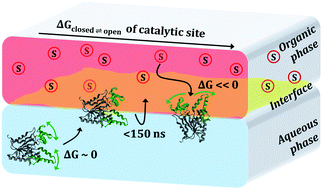
Lipases are water-soluble enzymes that catalyze the hydrolysis of lipids. Since lipids are mostly hydrophobic, lipase activity occurs preferentially at interfaces of aqueous and organic phases. In this work, we study the molecular mechanisms by which the Burkholderia cepacia lipase (BCL) is activated at interfaces of water with octane and with methyl caprylate (CAME). We show that BCL assumes very rapidly a preferential orientation at the interfaces, in which the active site is exposed to the organic phase. With BCL oriented to the interface, we compute the free energy of the aperture of the catalytic pocket using Adaptive Biasing Force MD simulations. The exposure to the organic phase promotes a clear stabilization of the open form of the catalytic pocket relative to the enzyme in water. This stabilization stems from the hydrophobicity of domains U1 and U2, which allows the penetration of organic solvents into the catalytic cleft impeding the closure of the pocket. Our results suggest that the structure and hydrophobicity of BCL are optimized for its activation in biphasic systems through the regulation of the accessibility of the catalytic pocket by, and for, hydrophobic substrates. The understanding of this mechanism may be useful for the design of proteins with targeted activation.


 Please wait while we load your content...
Please wait while we load your content...