Morphology-controlled syntheses of α-MnO2 for electrochemical energy storage
Abstract
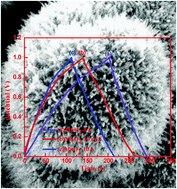
Manganese dioxide (MnO2) nanoarchitectures including microspheres assembled by nanosheets and hollow urchins assembled by nanorods have been successfully synthesized using a facile and efficient hydrothermal method at 150 °C. The effects of concentrations of the reactants and reaction time on the structures and morphologies of MnO2 were systematically investigated. The experimental results showed that the morphologies of MnO2 transformed into nanosheet-assembled microspheres (10 min) from nanorod-assembled hollow urchins (5 min) by tuning the suitable reaction time. The nanorod-assembled hollow urchins experienced the morphology transformation cycle from urchin to a disordered structure to urchin with the extension of the reaction time. Furthermore, the nanorods with different diameters and lengths were formed with different concentrations of reactants at the same reaction time (8 h). The MnO2 nanorods fabricated with 0.59 g KMnO4 showed a maximum specific capacitance (198 F g−1) with a good rate capability and excellent cycling stability (maintained 94% after 2000 cycles). Furthermore, the nanosheet-assembled microspheres exhibited the higher specific capacitance of 131 F g−1 at 1 A g−1 with a long-term cycling stability for the samples at different reaction times. These results indicated their promising applications as high-performance supercapacitor electrodes and provided a generic guideline in developing different nanostructured electrode materials for electrochemical energy storage.

 Please wait while we load your content...
Please wait while we load your content...