Enhancing the available specific surface area of carbon supports to boost the electroactivity of nanostructured Pt catalysts†
Abstract
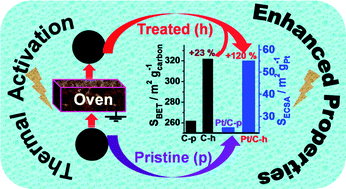
We report increasing improvements in the available specific surface area of the commonly used Vulcan XC 72R and Ketjenblack EC-600JD carbons by simple thermal pre-treatment. The treated Vulcan and Ketjenblack substrates have a specific surface area of 322 and 1631 m2 g−1, respectively, instead of 262 and 1102 m2 g−1 for the as-received materials, which is a 23 and 48% improvement. Subsequently, when used as platinum nanoparticle (3 nm) supports, the electrochemical active surface area is enhanced by factors of 2.2 and 1.2 for treated Vulcan and Ketjenblack carbons, respectively. Furthermore, electrochemical investigations have highlighted a surprisingly improved catalytic activity for the pre-treated Vulcan XC 72R and Ketjenblack EC-600JD supported Pt nanoparticles. In fact, the synthesized nanostructures from the so-called “Bromide Anion Exchange” method exhibit good catalytic activity toward glucose electrooxidation, both in the alkaline medium and the phosphate buffered solution at pH 7.4. More importantly, the present catalysts are four times more active than those in the literature prepared under similar conditions for glucose dehydrogenation at low potential (0.27 V vs. Reversible Hydrogen Electrode). Consequently, these remarkable trends uncovered herein provide ample new strategic routes for the pre-treatment of Vulcan XC 72R and Ketjenblack carbons for widespread uses.

 Please wait while we load your content...
Please wait while we load your content...