Transient UV pump–IR probe investigation of heterocyclic ring-opening dynamics in the solution phase: the role played by nσ* states in the photoinduced reactions of thiophenone and furanone†
Abstract
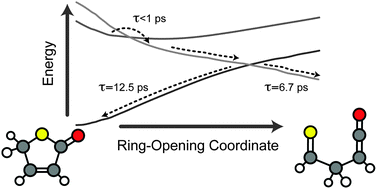
The heterocyclic ring-opening dynamics of thiophenone and furanone dissolved in CH3CN have been probed by ultrafast transient infrared spectroscopy. Following irradiation at 267 nm (thiophenone) or 225 nm (furanone), prompt (τ < 1 ps) ring-opening is confirmed by the appearance of a characteristic antisymmetric ketene stretching feature around 2150 cm−1. The ring-opened product molecules are formed highly vibrationally excited, and cool subsequently on a ∼6.7 ps timescale. By monitoring the recovery of the parent (S0) bleach, it is found that ∼60% of the initially photoexcited thiophenone molecules reform the parent molecule, in stark contrast with the case in furanone where there is less than 10% parent bleach recovery. Complementary ab initio calculations of potential energy cuts along the S–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and O–C(
O) and O–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) ring-opening coordinate reveals insights into the reaction mechanism, and the important role played by dissociative (n/π)σ* states in the UV-induced photochemistry of such heterocyclic systems.
O) ring-opening coordinate reveals insights into the reaction mechanism, and the important role played by dissociative (n/π)σ* states in the UV-induced photochemistry of such heterocyclic systems.

 Please wait while we load your content...
Please wait while we load your content...