Brønsted acids in ionic liquids: how acidity depends on the liquid structure
Abstract
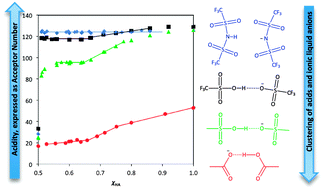
Gutmann Acceptor Number (AN) values have been determined for Brønsted acid–ionic liquid mixtures, over a wide compositional range. Four systems of general formula [C2mim][A]–HA (A− = bistriflamide, [NTf2]−; triflate, [OTf]−; mesylate, [OMs]−; or acetate, [OAc]−, [C2mim]+ = 1-ethyl-3-methylimidazolium cation) were studied. A library of Brønsted acidic systems of varying acidity was constructed and the AN parameter was found to be a convenient approach for quantifying their acidity. HOAc, HOMs and HOTf, when dissolved in ionic liquids, were found to associate with the respective anions to form hydrogen-bonded anionic clusters, [A(HA)x]−. In contrast, HNTf2 was solubilised as a discrete, undissociated molecule. AN values were sensitive to the presence of anionic clusters; acidity could be buffered to a particular AN by binding the solubilised acid in the anionic cluster form. Overall, a simple way to manipulate and quantify the Brønsted acidity of acid–ionic liquid mixtures was demonstrated, and measured AN values were related to liquid speciation.

 Please wait while we load your content...
Please wait while we load your content...