Ion formation pathways of crown ether–fullerene conjugates in the gas phase†
Abstract
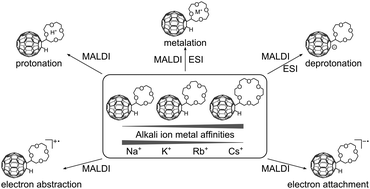
The ion formation of crown ether–[60]fullerene conjugates of the type (crown − H)–C60–H with crown = 12cr4, 15cr5 and 18cr6 has been studied with matrix-assisted laser desorption/ionisation (MALDI) and electrospray ionisation mass spectrometry (ESI MS). In total five different ways of ion formation are presented, including metalation (MALDI, ESI), protonation and oxidation (both in MALDI) in the positive-ion mode and deprotonation (MALDI, ESI) and reduction (MALDI) in the negative-ion mode. In line with thermochemistry, the deprotonation and electron transfer processes involve the C60 moiety as the charge-carrying entity, while protonation and metalation occur at the crown ether. Particular emphasis has been placed on the study of metal cation attachment in MALDI varying the crown ether size in the conjugate and using different alkali metal chlorides in the target preparation. Dissociation reactions of the metalated conjugates are influenced by the interaction strength of the metal cation to the crown ether fullerene conjugate. The data confirm an increase in bond strength with smaller metal cations, supporting the notion of charge density-driven interactions.

 Please wait while we load your content...
Please wait while we load your content...