A study into the extracted ion number for NASICON structured Na3V2(PO4)3 in sodium-ion batteries†
Abstract
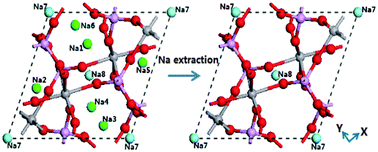
Excellent C-rate and cycling performance with a high specific capacity of 117.6 mA h g−1 have been achieved on NASICON-structure Na3V2(PO4)3 sodium-ion batteries. Two different Na sites, namely Na(1) and Na(2), are reported in the open three-dimensional framework, of which the ions at the Na(2) sites should be mainly responsible for the electrochemical properties. It is vitally important and interesting to find that there are two kinds of possible ion occupation of Na ions in Na3V2(PO4)3 and the investigation of ion-extraction number is firstly explored by discussing ion occupations with the help of first-principles calculations. The ion occupation of 0.75 for all Na sites is suitable for the configuration of [Na3V2(PO4)3]2, and the two-step extraction process accompanied by structure reorganization can account for the theoretical capacity of Na3V2(PO4)3.

 Please wait while we load your content...
Please wait while we load your content...