Diastereomeric preference of a triply axial chiral binaphthyl based molecule: a concentration dependent study by chiroptical spectroscopies†
Abstract
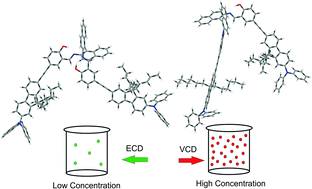
We have examined the effects of environmental perturbations, specifically solvents and concentrations, on axial chirality of a recently synthesized axially chiral binaphthyl fluorene based salen ligand, named AFX-155, {[2,2′-(1E,1′E)-(R)-1,1′-binaphthyl-2,2′-diylbis(azan-1-yl-1-ylidene)bis(methan-1-yl-1-ylidene)bis(4-((7-(diphenylamino)-9,9-dihexyl-9H-fluoren-2-l)ethynyl)-phenol)]}. Chirality and dominant conformations of AFX-155 in CDCl3 solvent have been characterized using vibrational absorption (VA) and vibrational circular dichroism (VCD) spectroscopy in combination with DFT calculations. AFX-155 exhibits triple axial chirality: one is at the binaphthyl ring and the other two are related to the axes of chirality along the –C–N bonds where Cs are part of the binaphthyl group. To evaluate solvent and concentration dependence, complementary VA and VCD experiments in both THF-d8 and CDCl3 have been performed, as well as the optical rotatory dispersion (ORD) and electronic CD (ECD) measurements in CDCl3 under much diluted conditions. While the binaphthyl chirality is determined by the synthetic route, the results show that the latter two axial chirality labels of the dominant diastereomers are concentration dependent. Under much diluted conditions, R-binaphthyl, R_intra_HB//R_extra_HB (R-RR) is favoured, whereas R-binaphthyl, S_intra_HB//S_extra_HB (R-SS) is the dominant species in a concentrated solution. This diastereomeric interconversion is found to be independent of the two solvents used. To provide insights into this interesting finding, conformational searches and the related spectral simulations have been carried out at the DFT/B3LYP/6-31G(d) level.

 Please wait while we load your content...
Please wait while we load your content...