Chemical nature of boron and nitrogen dopant atoms in graphene strongly influences its electronic properties†
Abstract
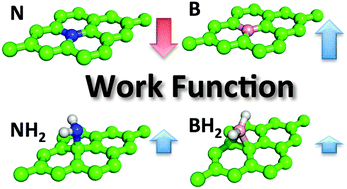
Boron and nitrogen doped graphenes are highly promising materials for electrochemical applications, such as energy storage, generation and sensing. The doped graphenes can be prepared by a broad variety of chemical approaches. The substitution of a carbon atom should induce n-type behavior in the case of nitrogen and p-type behavior in the case of boron-doped graphene; however, the real situation is more complex. The electrochemical experiments show that boron-doped graphene prepared by hydroboration reaction exhibits similar properties as the nitrogen doped graphene; according to theory, the electrochemical behavior of B and N doped graphenes should be opposite. Here we analyze the electronic structure of N/B-doped graphene (at ∼5% coverage) by theoretical calculations. We consider graphene doped by both substitution and addition reactions. The density of states (DOS) plots show that graphene doped by substitution of the carbon atom by N/B behaves as expected, i.e., as an n/p-doped material. N-doped graphene also has a lower value of the workfunction (3.10 eV) with respect to that of the pristine graphene (4.31 eV), whereas the workfunction of B-doped graphene is increased to the value of 5.57 eV. On the other hand, the workfunctions of graphene doped by addition of –NH2 (4.77 eV) and –BH2 (4.54 eV) groups are both slightly increased and therefore the chemical nature of the dopant is less distinguishable. This shows that mode of doping depends significantly on the synthesis method used, as it leads to different types of behaviour, and, in turn, different electronic and electrochemical properties of doped graphene, as observed in electrocatalytic experiments. This study has a tremendous impact on the design of doped graphene systems from the point of view of synthetic chemistry.

 Please wait while we load your content...
Please wait while we load your content...