Operating mechanisms of electrolytes in magnesium ion batteries: chemical equilibrium, magnesium deposition, and electrolyte oxidation†
Abstract
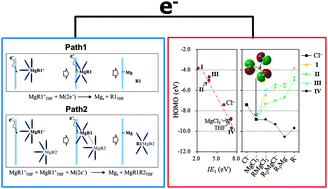
Since the early nineties there have been a number of reports on the experimental development of Mg electrolytes based on organo/amide-magnesium chlorides and their transmetalations. However, there are no theoretical papers describing the underlying operating mechanisms of Mg electrolytes, and there is no clear understanding of these mechanisms. We have therefore attempted to clarify the operating mechanisms of Mg electrolytes by studying the characteristics of Mg complexes, solvation, chemical equilibrium, Mg-deposition processes, electrolyte-oxidation processes, and oxidative degradation mechanism of RMgCl-based electrolytes, using ab initio calculations. The formation and solvation energies of Mg complexes highly depend on the characteristics of R groups. Thus, changes in R groups of RMgCl lead to changes in the equilibrium position and the electrochemical reduction and oxidation pathways and energies. We first provide a methodological scheme for calculating Mg reduction potential values in non-aqueous electrolytes and electrochemical windows. We also describe a strategy for designing Mg electrolytes to maximize the electrochemical windows and oxidative stabilities. These results will be useful not only for designing improved Mg electrolytes, but also for developing new electrolytes in the future.

 Please wait while we load your content...
Please wait while we load your content...