Sc2S@C68: an obtuse di-scandium sulfide cluster trapped in a C2v fullerene cage†
Abstract
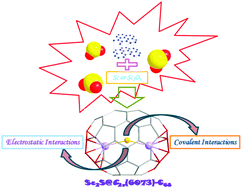
The spectrum-detected smallest sulfide clusterfullerene Sc2S@C68 has not been characterized yet. Herein, we explored a series of Sc2S@C68 species to determine which could be the most promising isomer. The results suggest that a sulfide cluster encapsulated in the C2v(6073)-C68 cage which violates the isolated pentagon rule (IPR) with two opposite pentalenes has the lowest energy and an overwhelming thermodynamic stability. Two scandium atoms coordinate with the two opposite pentalenes, leading to an obtuse Sc–S–Sc angle of 151°. The bond lengths of the two Sc–S bonds are equivalent. Frontier molecular orbital distributions exhibit substantial overlaps between metallic orbitals and cage orbitals, indicating that covalent interactions cannot be ignored, which have been unambiguously identified in terms of Mayer bond order and bonding critical point (BCP) indicator methods. Electrochemical properties as well as 13C NMR, UV-vis-NIR, and IR spectra of Sc2S@C2v(6073)-C68 have been theoretically studied to assist further experimental characterization.

 Please wait while we load your content...
Please wait while we load your content...