On the origin of ionicity in ionic liquids. Ion pairing versus charge transfer†
Abstract
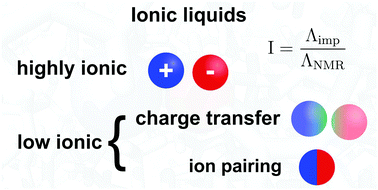
In this paper we show by using static DFT calculations and classical molecular dynamics simulations that the charge transfer between ionic liquid ions plays a major role in the observed discrepancies between the overall mobility of the ions and the observed conductivities of the corresponding ionic liquids, while it also directly suppresses the association of oppositely charged ions, thus the ion pairing. Accordingly, in electrochemical applications of these materials it is important to consider this reduction of the total charges on the ions, which can greatly affect the performance of the given process or device in which the ionic liquid is used. By slightly shifting from the salt-like to a molecular liquid-like system via the decreased charges, the charge transfer also fluidizes the ionic liquid. We believe that this vital information on the molecular level structure of ionic liquids offers a better understanding of these materials, and allows us to improve the a priori design of ionic liquids for any given purpose.

 Please wait while we load your content...
Please wait while we load your content...