An assessment of vapour pressure estimation methods†
Abstract
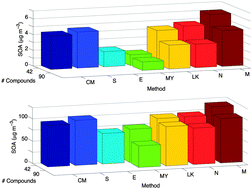
Laboratory measurements of vapour pressures for atmospherically relevant compounds were collated and used to assess the accuracy of vapour pressure estimates generated by seven estimation methods and impacts on predicted secondary organic aerosol. Of the vapour pressure estimation methods that were applicable to all the test set compounds, the Lee–Kesler [Reid et al., The Properties of Gases and Liquids, 1987] method showed the lowest mean absolute error and the Nannoolal et al. [Nannoonal et al., Fluid Phase Equilib., 2008, 269, 117–133] method showed the lowest mean bias error (when both used normal boiling points estimated using the Nannoolal et al. [Nannoolal et al., Fluid Phase Equilib., 2004, 226, 45–63] method). The effect of varying vapour pressure estimation methods on secondary organic aerosol (SOA) mass loading and composition was investigated using an absorptive partitioning equilibrium model. The Myrdal and Yalkowsky [Myrdal and Yalkowsky, Ind. Eng. Chem. Res., 1997, 36, 2494–2499] vapour pressure estimation method using the Nannoolal et al. [Nannoolal et al., Fluid Phase Equilib., 2004, 226, 45–63] normal boiling point gave the most accurate estimation of SOA loading despite not being the most accurate for vapour pressures alone.

 Please wait while we load your content...
Please wait while we load your content...