Alkali cation specific adsorption onto fcc(111) transition metal electrodes†
Abstract
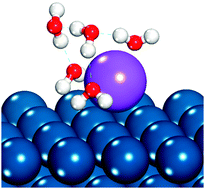
The presence of alkali cations in electrolyte solutions is known to impact the rate of electrocatalytic reactions, though the mechanism of such impact is not conclusively determined. We use density functional theory (DFT) to examine the specific adsorption of alkali cations to fcc(111) electrode surfaces, as specific adsorption may block catalyst sites or otherwise impact surface catalytic chemistry. Solvation of the cation–metal surface structure was investigated using explicit water models. Computed equilibrium potentials for alkali cation adsorption suggest that alkali and alkaline earth cations will specifically adsorb onto Pt(111) and Pd(111) surfaces in the potential range of hydrogen oxidation and hydrogen evolution catalysis in alkaline solutions.
- This article is part of the themed collection: Electrocatalysis - Fundamental Insights for Sustainable Energy

 Please wait while we load your content...
Please wait while we load your content...