Electrochemical characteristics of nanostructured platinum electrodes – a cyclic voltammetry study
Abstract
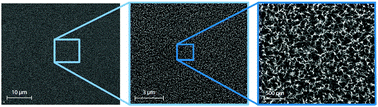
Platinum surfaces play a decisive role in catalysis in sensors, fuel cells, solar cells and other applications like neuronal stimulation and recording. Technical advances in nanotechnology contributed tremendously to the progress in these fields. A fundamental understanding of the chemical and physical interactions between the nanostructured surfaces and electrolytes is essential, but was barely investigated up to now. In this article, we present a wet-chemical process for the deposition of nanostructures on polycrystalline platinum surfaces. The electrochemically active surface area was increased by a factor of over 1000 times with respect to the geometrical surface. The influence of the nanostructures was examined in different acidic, alkaline, and neutral electrolytes. Comparing cyclic voltammograms of nanostructured and planar polycrystalline platinum revealed new insights into the microenvironment at the electrode–electrolyte interface. The characteristic features of the cyclic voltammograms were altered in their shape and strongly shifted with respect to the applied potential. In neutral buffered and unbuffered electrolytes the water window was expanded from 1.4 V to more than 2 V. The shifts were interpreted as local pH-changes and exhausted buffer capacity in direct proximity of the electrode surface due to the strong release and binding of protons, respectively. These polarized electrodes induce significant changes in the electrochemical potential of the electrolyte due to the high roughness of their surface. The electrochemical phenomena and the observed voltage shifts are crucial for the understanding of the basic mechanism at nanostructured electrodes and mandatory for designing fuel cells, sensors and many other devices.

 Please wait while we load your content...
Please wait while we load your content...