Solvent-induced conformational changes in cyclic peptides: a vibrational circular dichroism study†
Abstract
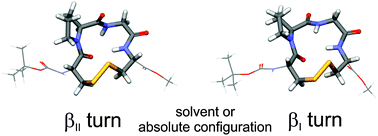
The three-dimensional structure of a peptide is strongly influenced by its solvent environment. In the present study, we study three cyclic tetrapeptides which serve as model peptides for β-turns. They are of the general structure cyclo(Boc-Cys-Pro-X-Cys-OMe) with the amino acid X being either glycine (1), or L- or D-leucine (L- or D-2). Using vibrational circular dichroism (VCD) spectroscopy, we confirm previous NMR results which showed that D-2 adopts predominantly a βII turn structure in apolar and polar solvents. Our results for L-2 indicate a preference for a βI structure over βII. With increasing solvent polarity, the preference for 1 is shifted from βII towards βI. This conformational change goes along with the breaking of an intramolecular hydrogen bond which stabilizes the βII conformation. Instead, a hydrogen bond with a solvent molecule can stabilize the βI turn conformation.

 Please wait while we load your content...
Please wait while we load your content...