Comparison of hydrogen production and electrical power generation for energy capture in closed-loop ammonium bicarbonate reverse electrodialysis systems†
Abstract
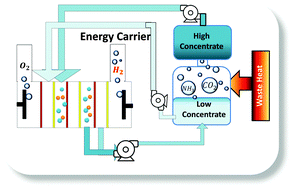
Currently, there is an enormous amount of energy available from salinity gradients, which could be used for clean hydrogen production. Through the use of a favorable oxygen reduction reaction (ORR) cathode, the projected electrical energy generated by a single pass ammonium bicarbonate reverse electrodialysis (RED) system approached 78 W h m−3. However, if RED is operated with the less favorable (higher overpotential) hydrogen evolution electrode and hydrogen gas is harvested, the energy recovered increases by as much ∼1.5× to 118 W h m−3. Indirect hydrogen production through coupling an RED stack with an external electrolysis system was only projected to achieve 35 W h m−3 or ∼1/3 of that produced through direct hydrogen generation.

 Please wait while we load your content...
Please wait while we load your content...