The nature of electronic excitations at the metal–bioorganic interface illustrated on histidine–silver hybrids†
Abstract
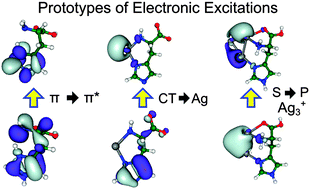
We present a joint theoretical and experimental study of the structure selective optical properties of cationic and anionic histidine–silver complexes with Ag and Ag3 which were prepared in the gas phase using mass spectroscopy coupled to electrospray ion source. Our TDDFT calculations provide general insight into the nature of electronic excitations at the metal–bioorganic interface that involve π–π* excitation within bioorganic subunits, charge transfer between two subunits and intrametallic excitations. The binding of silver to histidine, one of the most important amino acids, induces red shift in the optical absorption of protonated histidine particularly for anionic species. The presence of the smallest metallic subunit Ag3 increases the intensity of low energy transitions of histidine illustrating a metal cluster-induced enhancement of absorption of biomolecules in hybrid systems. Comparison of calculated absorption spectra with experimental photofragmentation yield provides structural assignment of the measured spectroscopic patterns. Our findings may serve to establish silver-labeling as the tool for the detection of histidine or histidine-tagged proteins.

 Please wait while we load your content...
Please wait while we load your content...