Hydrothermal synthesis and electrochemical properties of tin titanate nanowires coupled with SnO2 nanoparticles for Li-ion batteries†
Abstract
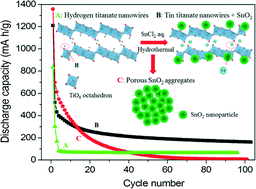
Tin titanate nanowires coupled with SnO2 nanoparticles have been prepared by combining hydrolysis of the Sn(II) precursor with tin-to-hydrogen ion exchange using layered hydrogen titanate nanowires as conformal templates under hydrothermal conditions. This synthetic strategy allows for incorporation of electrochemically active Sn into the layered titanate and simultaneous deposition of SnO2 nanoparticles on the as-prepared tin titanate nanowires. When used as anode materials in lithium ion batteries, the tin titanate nanowires coupled with SnO2 nanoparticles showed improved cycle performance and increased lithium storage capacity as compared with mesoporous SnO2 nanoparticle aggregates and hydrogen titanate nanowires. Electrochemical study indicated that introduction of SnO2 nanoparticles supported on tin titanate can buffer the large volume changes during the Li–Sn alloying and dealloying process in flexible layered titanate nanostructures with large interlayer distance. Besides, these composite structures exhibited remarkably low (<0.5 V) voltage for the Li insertion electrode in lithium ion batteries.

 Please wait while we load your content...
Please wait while we load your content...