When two symmetrically independent molecules must be different: “Crystallization-induced diastereomerization” of chiral pinanyl sulfone†‡
Abstract
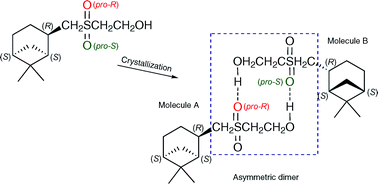
According to X-ray data, homochiral pinanyl sulfone crystallizes as an asymmetric dimer formed by pairwise H-bonds involving stereochemically different oxygen atoms of sulfonyl groups of molecules A and B. Thus, a pro-R atom is invoked for the construction of a relevant H-bond in molecule A, but in the case of molecule B only a pro-S atom is involved. Newly formed chiral sulfur atoms take opposite chirality in molecules A and B, while the configuration of the pinane skeleton remains unchanged. Such a stereochemical transformation is called “crystallization-induced diastereomerization”. The stability of the asymmetric dimer found in the crystal was evaluated within the framework of DFT (B3LYP, 6-31G (d,p)) and studied via IR spectroscopy in solution.

 Please wait while we load your content...
Please wait while we load your content...