Boron-nitride and aluminum-nitride “Pringles” and flapping motion†
Abstract
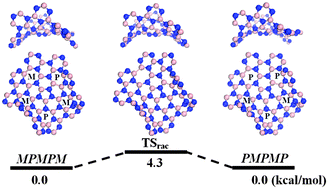
Motivated by the recent successful synthesis of a new nanocarbon, namely, a warped, double-concave graphene “Pringle” (Nat. Chem., 2013, 5, 739), we investigate properties of warped boron-nitride (BN) and aluminum-nitride (AlN) analogues, i.e., the non-planar B40N40H30 and Al40N40H30 “Pringles” using density functional theory (DFT) calculations. Particular attention is placed on the effect of non-hexagonal rings on the stability and physical properties of BN and AlN Pringles. We find that the warped BN and AlN Pringles with one pentagon and five heptagons are stable without imaginary frequencies. Both the warped B40N40H30 and Al40N40H30 Pringles are expected to be flexible in solution as both can periodically change their shape in a dynamic “flapping” fashion due to their much lower activation barrier of racemization compared to that of the C80H30 counterpart. Since the warped B40N40H30 possesses a smaller HOMO–LUMO gap than the planar B39N39H30, it is expected that incorporating non-hexagonal ring defects by design can be an effective way to modify electronic properties of BN-based nanoplates.

 Please wait while we load your content...
Please wait while we load your content...