Stabilization of β-peptide helices by direct attachment of trifluoromethyl groups to peptide backbones†
Abstract
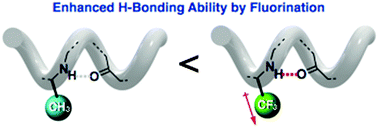
The 14-helix structure of oligo-β-peptides was significantly stabilized by direct attachment of CF3 groups to their backbones. Our studies indicate that this stabilization originates from the CF3-promoted increase in the intramolecular hydrogen-bonding ability of their backbone amides, leading to a novel strategy to stabilize peptide folding.

 Please wait while we load your content...
Please wait while we load your content...