Expanding the limit of Pd-catalyzed decarboxylative benzylations†
Abstract
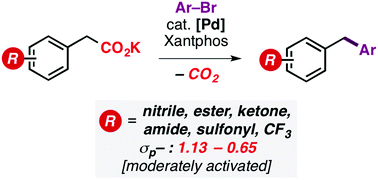
The Pd-catalyzed decarboxylative cross-coupling of electron-deficient aryl acetates with aryl bromides is reported. The method widens the scope of benzylic partners that can undergo efficient reactivity from highly activated nitrophenylacetates established previously, to a diverse series of substrates bearing modestly stabilizing groups, allowing direct access to functionalized diarylmethanes. Mechanistic studies support the role of dienolates as key intermediates in the coupling process.
- This article is part of the themed collection: 2018 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...