Mild access to planar-chiral ortho-condensed aromatic ferrocenes via gold(i)-catalyzed cycloisomerization of ortho-alkynylaryl ferrocenes†
Abstract
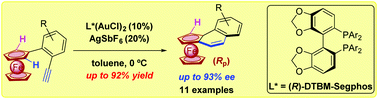
An efficient approach to (Rp) planar-chiral tri- and tetracyclic ortho-condensed aromatic ferrocenes was developed through the enantioselective cationic Au(I)-catalyzed cycloisomerization, in the presence of bidentate phosphine ligand (R)-DTBM-Segphos, from readily available ortho-alkynylaryl ferrocenes under very mild conditions (11 examples, up to 92% yield and 93% ee).

 Please wait while we load your content...
Please wait while we load your content...