Inducing achiral aliphatic oligoureas to fold into helical conformations†
Abstract
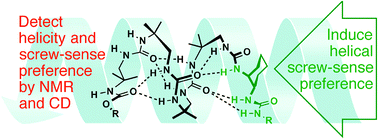
The ability of urea-linked oligomers of achiral diamines (achiral analogues of the well-established chiral oligourea foldamers) to adopt helical conformations was explored spectroscopically. Up to four achiral units were ligated either to a well-formed helical trimer or to a single chiral diamine, and the extent to which they adopted a screw-sense preference was determined by NMR and CD. In the best performing cases, a trimeric chiral oligourea and even a single cis-cyclohexanediamine monomer induced folding into a helical conformation.

 Please wait while we load your content...
Please wait while we load your content...