A general and practical palladium-catalyzed monoarylation of β-methyl C(sp3)–H of alanine†
Abstract
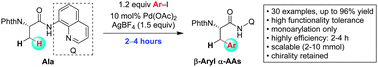
A palladium-catalyzed monoarylation of β-methyl C(sp3)–H of an alanine derivative with aryl iodides using an 8-aminoquinoline auxiliary is described. The reaction is highly efficient, scalable and compatible with a variety of functional groups with complete retention of chirality, providing a general and practical access to various β-aryl-α-amino acids. The synthetic potential of this protocol is further demonstrated in the sequential synthesis of diverse β-branched α-amino acids.
- This article is part of the themed collection: 2015 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...