Studies towards the synthesis of halomon: asymmetric hexafunctionalisation of myrcene†
Abstract
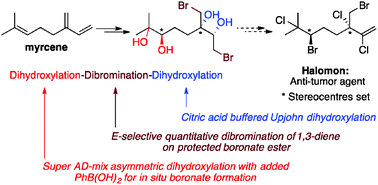
A novel dihydroxylation–dibromination–dihydroxylation sequence employing in situ protection of diols as boronate esters during the dihydroxylation reactions provides the first enantiomerically pure hexafunctionalised myrcene derivative. This concise four-step asymmetric sequence provides an advanced intermediate for the targeted synthesis of halomon via stereospecific transformations, where both stereogenic centres of the natural product have been set.

 Please wait while we load your content...
Please wait while we load your content...