A Mannich/cyclization cascade process for the asymmetric synthesis of spirocyclic thioimidazolidineoxindoles†
Abstract
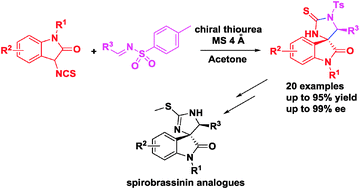
An asymmetric cascade Mannich/cyclization reaction between 3-isothiocyanato oxindoles and sulfimides using a commercially available organocatalyst has been developed. A wide range of structurally diverse spiro[imidazolidine-4,3′-oxindole] derivatives were obtained with good yields (up to 92%) and excellent enantioselectivities (up to 99% ee).

 Please wait while we load your content...
Please wait while we load your content...