Ionophore-based ion-exchange emulsions as novel class of complexometric titration reagents†
Abstract
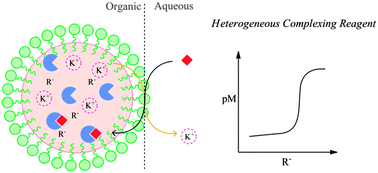
Complexometric titrations rely on a drastic change of the pM value at the equivalence point with a water soluble chelator forming typically 1 : 1 complexes of high stability. The available chemical toolbox of suitable chelating compounds is unfortunately limited because many promising complexing agents are not water soluble. We introduce here a novel class of complexometric titration reagents, a suspension of polymeric nanospheres whose hydrophobic core is doped with lipophilic ion-exchanger and a selective complexing agent (ionophore). The emulsified nanospheres behave on the basis of heterogeneous ion exchange equilibria where the initial counter ion of the ion-exchanger is readily displaced from the emulsion for the target ion that forms a stable complex in the nanosphere core. Two different examples are shown with Ca2+ and Pb2+ as target ions. The lack of protonatable groups on the calcium receptor allows one to perform Ca2+ titration without pH control.

 Please wait while we load your content...
Please wait while we load your content...