N-Heterocyclic carbene-catalyzed double acylation of enones with benzils†
Abstract
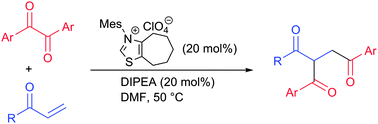
Thiazolium carbene-catalyzed reaction of aromatic 1,2-diketones with enones in aprotic solvents gave double acylation products in good yields, whereas hydroacylation products formed by Stetter reaction were not detected at all. These results suggested the generation of aroyloxyenamine species from the 1,2-diketones instead of hydroxyenamines (Breslow intermediates).

 Please wait while we load your content...
Please wait while we load your content...