Stereodefined acyclic trisubstituted metal enolates towards the asymmetric formation of quaternary carbon stereocentres†
Abstract
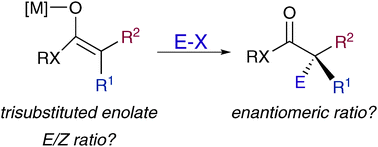
Reactions that involve metal enolate species are amongst the most versatile carbon–carbon bond forming processes available to synthetic chemists. Enolate species are involved in a multitude of powerful applications in asymmetric organic synthesis, but the generation of fully substituted enolates in a geometrically defined form is not easily achieved especially in acyclic systems. In this Feature Article we focus on the most prominent examples reported in the literature describing the formation of highly diastereo- and enantiomerically enriched quaternary stereocentres in acyclic molecules derived from stereodefined non-cyclic trisubstituted metal enolates.

 Please wait while we load your content...
Please wait while we load your content...