Native chemical ubiquitination using a genetically incorporated azidonorleucine†
Abstract
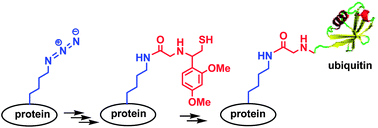
A robust chemical ubiquitination method was developed. The method employed a genetically incorporated azidonorleucine as an orthogonal lysine precursor for the installation of a Gly residue bearing an Nα-auxiliary which mediated the ligation between ubiquitin(1–75)-thioester and the target protein. To demonstrate our methodology, a model protein, K48-linked diubiquitin, was synthesized with an overall yield of 35%.

 Please wait while we load your content...
Please wait while we load your content...