Surfactant-free CO2-based microemulsion-like systems†
Abstract
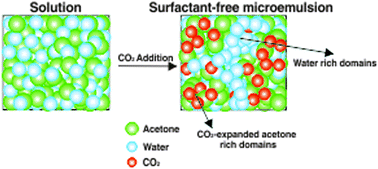
The presence of water-rich and water-lean nanodomains in a transparent, pressurized “water–acetone–CO2” mixture was revealed by Raman spectroscopy. This nano-structured liquid can be classified as a surfactant-free microemulsion-like system and has the capacity to dissolve hydrophobic compounds, such as ibuprofen, in the presence of large amounts of water. This finding opens new opportunities in the fields of confined reactions and material templating.

 Please wait while we load your content...
Please wait while we load your content...