Abstract
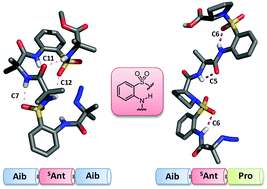
This communication describes the influence of β-aminobenzenesulfonic acid (SAnt) on the conformational preferences of hetero foldamers. The designed (Aib-SAnt-Aib)n and (Aib-SAnt-Pro)n oligomers display a well-defined folded conformation featuring intramolecular mixed hydrogen bonding (7/11) and intra-residual (6/5) H-bonding interactions, respectively.

 Please wait while we load your content...
Please wait while we load your content...