From thioether substituted porphyrins to sulfur linked porphyrin dimers: an unusual SNAr via thiolate displacement?†
Abstract
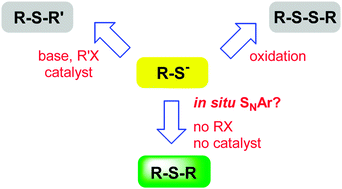
Treatment of meso 2-ethylhexyl-3-mercaptopropionate substituted porphyrins with base at room temperature generated a porphyrin thiolate anion which in situ reacted in a nucleophilic aromatic substitution (SNAr) reaction with remaining thioether derivative. This reaction yielded S-linked bisporphyrins in good yields, with mechanistic insight obtained via displacement reactions. Additionally, SNAr of the thioether chain was achieved using S- and organolithium nucleophiles.

 Please wait while we load your content...
Please wait while we load your content...