Conformational distribution of surface-adsorbed fibronectin molecules explored by single molecule localization microscopy†
Abstract
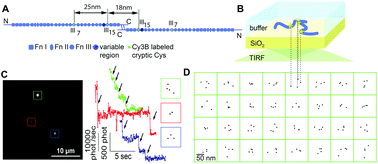
Adsorbed proteins that promote cell adhesion mediate the response of cells to biomaterials and scaffolds. As proteins undergo conformational changes upon surface adsorption, their functional display may be significantly affected by surface chemistry or solution conditions during the adsorption process. A high-resolution localization microscopy technique is extended here to probe the conformation of individual fibronectin (Fn) molecules at the glass–water interface under physiological buffer conditions. To map distances, four available cysteines located on the modules FnIII7 and FnIII15 of dimeric Fn were site-specifically labeled with Cy3B, and their relative positions were determined by stepwise photobleaching with nanometer precision. The four labels on single Fn molecules did not show a uniform or linear arrangement. The distances between label positions were distributed asymmetrically around 33 nm with a tail towards higher distances. Exposure of Fn to denaturing solution conditions during adsorption increased the average distances up to 43 nm for 4 M guanidinium HCl, while changing the solution conditions after the adsorption had no effect, indicating that the observed intra-molecular distances are locked-in during the adsorption process. Also surface coatings of different hydrophobicity altered the conformational distribution, shifting label distances from a median of 24 nm on hydrophilic to 49 nm on hydrophobic surfaces. These results further highlight that the conformation of macromolecules at interfaces depends on the adsorption history. While illustrated here for surface adsorbed Fn, the power of localization-based microscopy extends the repertoire of techniques to characterize biomolecules at interfaces.

 Please wait while we load your content...
Please wait while we load your content...