Determination of thirteen antibiotics in drug products – A new LC-MS/MS tool for screening drug product quality†
Abstract
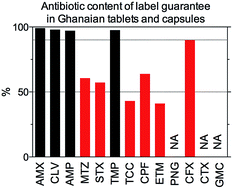
Poor quality antibiotic medicines in circulation in Sub-Saharan Africa continue to be a burden. Pharmaceutical trade in substandard and counterfeit medicines is on the rise. The chemical quality of antibiotics dispensed in health facilities and recognised drug outlets in Ghana, when compromised, could be a major drawback to efforts made in fighting antibiotic resistance globally. To improve on antibiotic drug quality monitoring, a liquid chromatography-tandem mass spectrometry (LC-MS/MS) methodology, which is capable of quantifying thirteen antibiotics in drug products, was developed and validated in present work. The methodology was applied to various drug products including tablets, capsules, suspensions, syrups, intravenous and injection solutions as well as ear and eye droplets used as essential medicines in a Sub-Saharan country, Ghana.

 Please wait while we load your content...
Please wait while we load your content...