Mesoporous titania thin films as efficient enzyme carriers for paraoxon determination/detoxification: effects of enzyme binding and pore hierarchy on the biocatalyst activity and reusability
Abstract
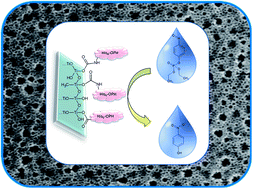
In this work we demonstrate the efficient immobilization of histidine 6-tagged organophosphate hydrolase (His6–OPH), an organophosphate-degrading enzyme, on mesoporous titania thin films. This permits the use of the biocatalyst films as efficient tools in the detection/detoxification of paraoxon. His6–OPH was immobilized on mesoporous thin films with uniform (9 nm) and bimodal (13–38 nm) pore size distribution, through covalent attachment and physical adsorption. The biocatalyst films show good activity, and enhanced stability with respect to the free enzyme at extreme conditions of pH and temperature, especially around neutral pH and room temperature. In addition, the bioactive films can be easily separated from the reaction media and reused multiple times without significant loss of activity.

 Please wait while we load your content...
Please wait while we load your content...