High resolution magic angle spinning NMR as a tool for unveiling the molecular enantiorecognition of omeprazole by amylose-based chiral phase
Abstract
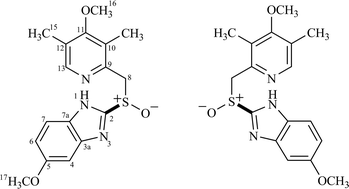
Polysaccharide-based chiral stationary phases (CSP) demonstrate great versatility and higher chiral selectivity for a variety of chiral compounds in multimodal elution modes (normal, reverse and polar organic). The main role of CSP phenyl carbamate based derivatives as chiral selectors is the formation of diastereoisomeric complexes by means of π–π interaction, dipole–dipole, hydrogen bonding and/or inclusion complex mechanisms. Nevertheless, the mechanism behind their enantioselectivity requires clarification. High resolution magic angle spinning nuclear magnetic resonance spectroscopy (1H HR/MAS NMR) has provided key information on the recognition process at the binding sites of the CSP surface. Herein we report the results obtained using omeprazole as a probe for these investigations.
- This article is part of the themed collection: Analytical Sciences in Brazil

 Please wait while we load your content...
Please wait while we load your content...