Bisphenol A determination in baby bottles by chemiluminescence enzyme-linked immunosorbent assay, lateral flow immunoassay and liquid chromatography tandem mass spectrometry
Abstract
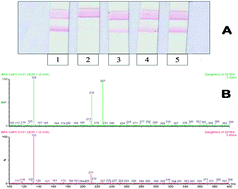
Two immunoassays, a Lateral Flow ImmunoAssay (LFIA) based on colloidal gold nanoparticle labels and an indirect competitive chemiluminescence enzyme-linked immunosorbent assay (CL-ELISA), were developed and a high performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was optimized to assess the possible release of bisphenol A (BPA, 4,4′-isopropylidenediphenol) from different plastic baby bottles treated with simulating solutions. Coating conjugate concentration, anti-BPA antibody dilution, incubation time of the primary and secondary antibodies, and tolerance to different organic solvents were optimized to obtain the best performance of the ELISA with chemiluminescent end-point detection. The influence of different buffers on LFIA performance was also evaluated. Both methods showed good repeatability (mean CV value around 13%) and sensitivity. Reproducibility tests for CL-ELISA gave a mean CV value of about 25%. The IC50 and Limit of Detection (LOD) values of CL-ELISA were 0.2 and 0.02 ng mL−1, respectively. The LOD of LFIA was 0.1 μg mL−1. A LC-MS/MS method was also optimized. The separation was performed in a C18 column with a triple-quadrupole mass spectrometer with electrospray ionisation interface. The method showed a good linearity in the range 2 to 500 ng mL−1, with a regression coefficient of 0.998. In the simulating solutions the detection and quantification limits, calculated by the signal to noise level of 3 (S/N = 3), were 5.8 ng mL−1 and 17.4 ng mL−1, respectively. This limit of quantification was about 3 and 35 times lower than the permitted limits set by the official method CEN/TS 13130-13 (0.05 μg mL−1) and by the Directive 2004/19/EC (0.6 μg mL−1), respectively. The methods were applied to determine BPA release from baby bottles, performing repeated procedures according to EU and national regulations. The results demonstrated that no BPA migration from the tested plastic materials occurred with only one exception. The migrated amount, above the regulatory limits, was detected by all the mentioned assays.

 Please wait while we load your content...
Please wait while we load your content...