Highly efficient deep-blue organic electroluminescent devices (CIEy ≈ 0.08) doped with fluorinated 9,9′-bianthracene derivatives (fluorophores)
Abstract
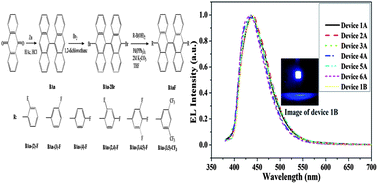
A series of new fluorinated 9,9′-bianthracene derivatives (BAnFs) have been designed and synthesized to serve as deep-blue dopants in organic electroluminescent (EL) devices. With the different substitution patterns of the electron-withdrawing groups, such as F and CF3, the photophysical properties, the energy levels and thermal stability of these BAnFs are tuned, which are supported by a density functional study of their geometry and electronic structure. In the thin film state, the fluorescent emissions of the BAnFs are fine-tuned from 448 to 439 nm, with varied fluorinated phenyl rings attached to the 9,9′-bianthracene core. All the BAnFs show a considerable thermal stability, which have high Tg values, above 150 °C. A pure blue emission at the Commission Internationale de l'Éclairage (CIE) coordinates (0.156, 0.083), has been achieved using the host 4,4′-bis(N-carbazolyl)biphenyl (CBP) doped with 10,10′-bis(3,5-bis(trifluoromethyl)phenyl)-9,9′-bianthracene (BAn-(3,5)-CF3). The maximum current efficiency and power efficiency of the BAn-(3,5)-CF3-doped device are 3.05 cd A−1 and 2.62 lm W−1, corresponding to 5.02% of the maximum external quantum efficiency. The synthesized new fluorinated 9,9′-bianthracene derivatives show potential applications as highly efficient pure blue emitters for organic light emitting devices.

 Please wait while we load your content...
Please wait while we load your content...