Water adsorption effects of nitrate ion coordinated Al2O3 dielectric for high performance metal-oxide thin-film transistor†
Abstract
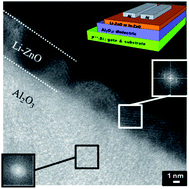
A solution-processed ionic amorphous Al2O3 dielectric with a low temperature annealing process at 350 °C shows good compatibility and high performance in metal oxide semiconductor thin film transitors (TFTs) such as Li–ZnO TFTs and In–ZnO TFTs. The Li–ZnO/Al2O3 and In–ZnO/Al2O3 TFTs, with solution-processability and low temperature annealing at a maximum of 350 °C, exhibited field-effect mobilities of 46.9 cm2 V−1 s−1 in crystalline Li–ZnO/Al2O3 TFTs and 44.2 cm2 V−1 s−1 in amorphous In–ZnO/Al2O3 TFTs with an on/off current ratio of more than 105. The proton mobile ion, such as hydrogen ion (H+) from chemisorbed water, in the ionic Al2O3 dielectric remarkably induces a high performance capacitance by the formation of an electrical double layer. The chemisorbed water was monitored by FT-IR and ellipsometric porosimetry measurements. Furthermore, the addition of H2O2 to the ionic Al2O3 dielectric precursor successfully suppressed the oxygen vacancies in the dielectric layer, which caused the electrical trap and pass, and confirmed the stable operation. These ionic amorphous Al2O3 dielectrics show good potential as switching TFTs devices in advanced displays, because they can satisfy the various demands of next-generation high-performance TFTs, such as low-cost, solution-processability, and a relatively low-temperature process.

 Please wait while we load your content...
Please wait while we load your content...