Cross-linking of discotic tetraazaporphyrin dyes in 2 and 3 dimensions by “click” chemistry†
Abstract
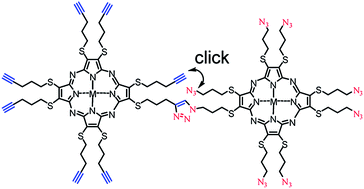
An intrinsic shortcoming of self-organizing materials is their susceptibility to structural changes by mechanical forces and exposure to chemicals and radiation. Cross-linking of the molecules in the desired supramolecular structure is a generally applicable pathway to structurally more stable materials but is difficult to apply to self-organizing materials because the introduction of cross-linkable groups affects their self-organization and the process of cross-linking may alter the supramolecular structure of the preceding phase. Reported here are the synthesis, thermal properties, and cross-linking of octaalkylthio substituted tetraazaporphyrins that contain either eight terminal azide groups or eight terminal acetylene groups. Synthesis of these compounds requires the preparation of the azide and alkyne containing side-chains and their reaction with 1,2-dicyanoethylene-1,2-dithiolate to the corresponding maleodinitrile intermediates. All maleodinitriles are successfully converted to tetraazaporphyrins by the established Mg templated cyclotetramerization in typical yields of 60–70%. Thermal properties of the metal-free and copper(II) metallated tetraazaporphyrins were studied by thermal gravimetric analysis, polarized optical microscopy, differential scanning calorimetry, and variable temperature powder X-ray diffraction measurements. Azide substituted tetraazaporphyrins with trimethylene and hexamethylene spacers as well as acetylene derivatives with trimethylene spacers unexpectedly display columnar mesophases over ranges of temperature from 30 °C to 110 °C. A thermally activated cross-linking of a hexagonal columnar mesophase by cycloaddition at 65 °C is demonstrated for a 1 : 1 mixture of azide and acetylene derivatives. At this temperature the reaction progresses for up to 48 hours but renders the mesophase insoluble and stable to above 200 °C. The structure of the mesophase is surprisingly little affected by the cross-coupling process that reaches a conversion of 60% of all azide and acetylene groups based on IR measurements. Conversion of up to 80% of azide and acetylene groups is reached by copper catalysed cross-linking of a 1 : 1 mixture in solution to generate insoluble polymers. A similar degree of conversion is achieved by copper catalysed cross-linking of a 1 : 1 mixture as Langmuir film after 3 hours. However, transfer of intact cross-linked Langmuir films onto substrates was not successful.

 Please wait while we load your content...
Please wait while we load your content...