Rational surface modification of Mn3O4 nanoparticles to induce multiple photoluminescence and room temperature ferromagnetism†
Abstract
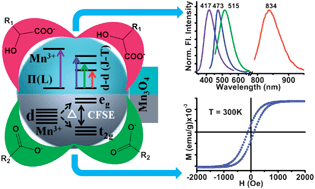
Surface modification can have a significant influence on the materials behavior at the nanoscale and can lead to nanostructures with novel properties. Here, we demonstrate the surface modification induced multiple photoluminescence and room temperature ferromagnetic activation of Mn3O4 nanoparticles (NPs). Employing a systematic variation of the ligands, their functional groups and the structural position of the functional groups, we have identified the necessary and sufficient structural requirements of the surface co-ordinating ligands, in order to induce unprecedented optical/magnetic responses from the NPs. Using a multitude of spectroscopic techniques, we have investigated the mechanism behind the emergence of the multiple photoluminescence (PL), and it is revealed that the presence of a α-hydroxy carboxylate moiety in the ligands is necessary to activate the Jahn–Teller (J–T) splitting of Mn3+ ions on the NP surface and the corresponding d–d transitions along with the ligand-to-metal charge transfer transitions (LMCT, associated with Mn2+/3+–ligand interactions) is the key factor. However, the presence of a carboxylate group on the surface coordinating ligands is sufficient to activate the room temperature ferromagnetism of the NPs. Moreover, it has been observed that the ligands that induced the smallest crystal field splitting energy (CFSE) resulted in the strongest ferromagnetic activation of the NPs. Finally, the functionalized material has been identified as an efficient catalyst for the photo-degradation of a model cationic organic dye. Apart from the fundamental scientific interest, these results represent a promising route for the rational design of Mn3O4 NPs adaptable to diverse applications.

 Please wait while we load your content...
Please wait while we load your content...